Welcome to Part IV of our four-part blog series, The Life and Times of a Fire Investigator. Read Part III here.
The previous three installments of this series have covered the fundamental building blocks of the fire investigation profession; this entry delves into a case study which ties everything together.
They say that one or two fire scenes define an investigators career. Without a doubt, the event I’m about to describe was the defining moment of my own. I’ll present the situation as I experienced it, including accompanying photos taken during my investigation, alongside notable fire code changes which resulted.
September 19, 2011
The summer was coming to an end, and the weather was mild and pleasant. However, things were about to heat up.
Sometime around 7:00 a.m., a fire began inside a dental office in Spokane Valley and, within minutes, triggered a large explosion. Glass and debris launched into the parking lot, and a large chunk of concrete fell from the side of the building. Luckily, no one was hurt; the one employee in the office at the time made it outside without incident.
I arrived on the scene that morning and it soon became clear what had happened.
The initial fire broke out in the storage room for oxygen and nitrous oxide tanks. Nitrous oxide, commonly used in dental practices, is a strong oxidizer that, like oxygen, causes materials to ignite more easily and burn far more rapidly. Seven tanks were placed against the southern and western walls. Three of these tanks were nitrous oxide and four were oxygen.
Opposite the tanks on the eastern wall, a simple wireframe storage rack held a variety of combustible items.
The storage room had rated metal doors and fire rated walls; ultimately, they did little to restrain the force the blast.
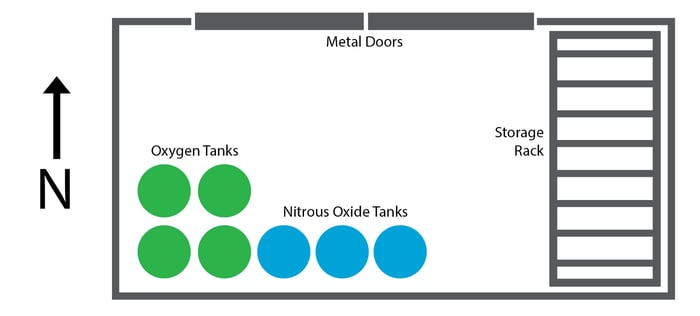
Fire broke out amidst the combustible items on the storage rack. As it grew, it began to heat up the side of the easternmost nitrous oxide tank. 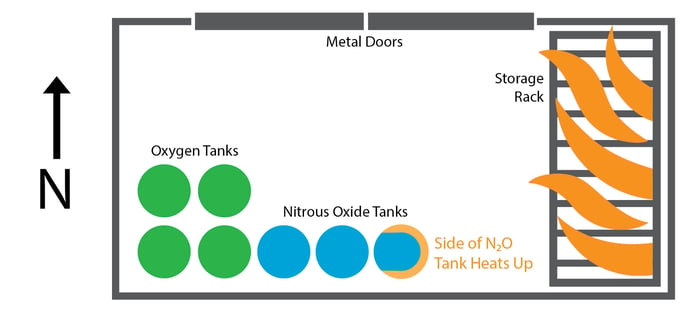
Nitrous oxide is a liquid under pressure; when heat is applied, it starts to boil. In this case, the pressure in the tank rapidly increased to the point where the tank violently ruptured. This type of tank failure is called a boiling liquid expanding vapor explosion (BLEVE). At a normal fire scene, you will see propane tanks, paint cans, or even lighters explode in this way.
The size of the tank and the fact that it was full at the time of the fire increased the explosions intensity, generating an explosion akin to that of high explosives, such as dynamite.
Total destruction
The resulting explosion was devastating, destroying much of the ground floor dental office. Here are some pictures of the resulting destruction:

View from outside the office building.
 Two photos taken immediately outside the remains of the medical gas storage room. As you can see on the left, several scorched tanks are laying on their side.
Two photos taken immediately outside the remains of the medical gas storage room. As you can see on the left, several scorched tanks are laying on their side.

The rated metal door to the medical gas storage room.

Remnants of the gas tanks.
While the immediate vicinity of the medical gas storage room took the greatest hit, much of the floor where the dental office was located sustained massive amounts of damage. Windows were blown out and rooms destroyed. It seemed that all that was left of a once functioning office was twisted metal and charred debris.
Dental offices are not supposed to explode, so what happened?
Aftermath
My investigation into the explosion determined one thing for certain: the fire started in the storage room. Furthermore, it was my determination that a faulty oxygen system was primarily responsible for the conflagration and resulting explosion.
Oxygen promotes combustion; without it, fire cannot start. The higher the concentration of oxygen in the air, the easier it is for materials to combust and spread. In fact, many materials that would otherwise not be combustible can ignite in an oxygen enriched atmosphere. Even a small increase (example: 3% increase, from 21% to 24%) can create a volatile situation.
Without proper maintenance, gas storage tanks degrade and leak. An oxygen leak can enrich the atmosphere and create an environment in which ignition is easier to achieve, especially in confined spaces. In oxygen enriched environments, even the slightest spark can result in ignition.
But oxygen alone does not a fire make.
Looking back to those moments before the explosion occurred, we have a medical gas storage room enriched with oxygen and a storage rack loaded with combustible material; all that's left is the source of ignition.
Let's explore a number of ways the fire could have started:
Mechanical impact
Heat is produced when two objects strike one another. For example, a mechanical component may break or fall off, producing heat upon impact.
Particle impact
Small particles within a flowing oxygen stream can strike a surface within the oxygen system, heat up, and ignite larger materials.
Friction
Two solid materials rubbing against one another can generate heat. Oxygen traveling in a closed system can create the same effect.
Compression heating
Gas flowing through an opening from high to low pressure expands and accelerates. When the flow becomes blocked, the gas recompresses, reaching a heat level capable of causing ignition. 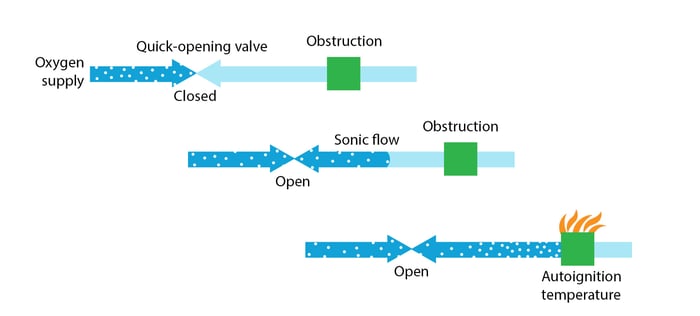
Insufficient code
Regardless of how ignition occurred, the oxygen enriched atmosphere created within the medical gas storage room created favorable conditions for fire ignition. Proper maintenance and inspection of oxygen systems could have prevented these conditions from occurring in the first place.
So I checked the fire code to see what rules and regulations were in place. What I found surprised me.
In 2009, Washington adopted a new version of the International Fire Code (IFC). During the review process, an amendment was put forth, stating:
All distribution piping, supply manifolds, connections, regulators, valves, alarms, sensors, and associated equipment shall be in accordance with the Plumbing Code.
This amendment also removed IFC Section 3006.4, the reference to NFPA 99 that required inspections and testing annually. At the time, it was thought that NFPA 99 would conflict with the Plumbing Code.
IFC 3006.4: Medical gas systems including, but not limited to, distribution piping, supply manifolds, connections, pressure regulators and relief devices and valves, shall comply with NFPA 99 and the general provisions of this chapter

NFPA 99 is the Health Care Facilities Code, designed to ensure health care safety and protect patients, staff, and visitors from dangers. Let's look at what NFPA 99 says about medical gas systems:

So, in 2011, at the time of the explosion, the current Washington IFC did not have a clause mandating inspection and maintenance on medical gas systems.
I had only just discovered that a lack of inspection and maintenance may very well be the primary cause of a devastating explosion which destroyed an entire dental office and could very well have cost people their lives. Something had to be done.
State Building Code Council (SBCC)
The SBCC is a state agency created by the legislature to provide independent analysis and objective advice to the legislature and the Governor's Office on state building code issues. The primary function of the SBCC is to maintain and amend building codes across the state of Washington. To this end, the review and amendment process involves a fire code technical advisory group, or TAG. Members of TAG review code change proposals, conduct research, and identify impacts of code alteration.
I petitioned the SBCC and TAG to consider adopting the criteria for medical gas systems laid out in NFPA 99 The result, following extensive review, was adoption of the following code:
5306.4 Medical gas systems. The maintenance and testing of medical gas systems including, but not limited to, distribution piping, supply manifolds, connections, pressure regulators and relief devices and valves, shall comply with the maintenance and testing requirements of NFPA 99 and the general provisions of this chapter.
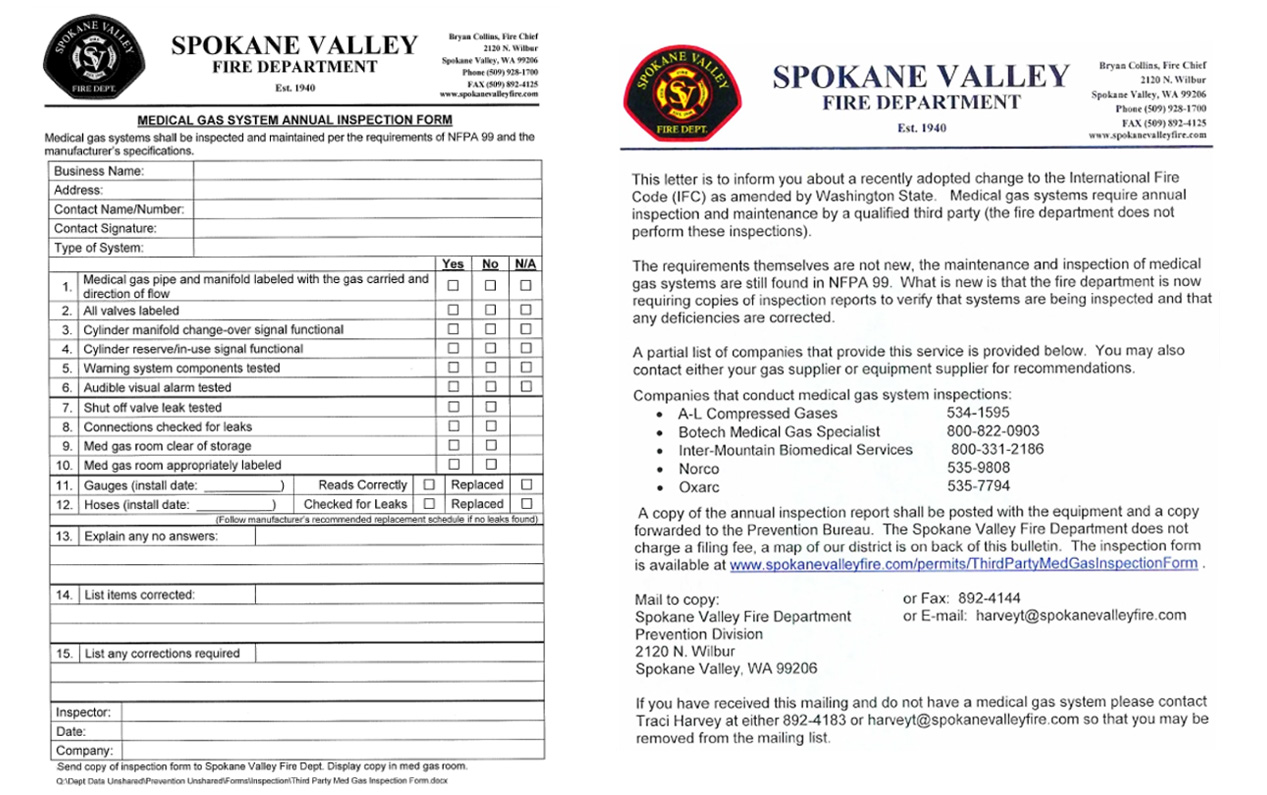
This change to the IFC created a system in which fire departments require proof of inspection across the state.
Conclusion
No matter how inconsequential a single line of fire code may seem, each line furthers our goal of protecting people and property. I'm very proud to have been involved in the process of making the IFC stronger than it was previously.
"The Life and Times of a Fire Investigator" blog series is now complete. I've enjoyed sharing with you my experience and experiences, giving a peek behind the curtain into the world of fire investigation.
If you'd like to check out previous parts of this series, click here.











